What Does It Mean for an Equation to Be Balanced
The law of conservation of mass governs the balancing of a chemical equation. They are balanced by adding suitable coefficients without changing the molecular formula of reactants and products.

How To Balance Chemical Equations Chemical Equation Equations Teaching Science
So to say an equation is balanced means that there there there is the same number of Adams on both sides of the equation.

. Thus the symbol 2 NaHCO3 indicates two units of sodium bicarbonate which contain 2 Na atoms 2 H atoms 2 C atoms and 6 O atoms 2 X 3 6 the coefficient times the subscript for O. When an equation is balanced it means the number of atoms of each element in the reactant side is equal to that of the product side. An equation is said to be balanced when the number of atoms of each element is the same on both sides of the reaction reactant side and product side.
A chemical equation is balanced when the number of each kind of atom is the same on both sides of the reaction. The equal number of atoms from both sides of the equation reactants and products follow the Law of Conservation of Mass. Lets take a look at an equation representing.
It truly is the number one factor in solving equations. A balanced equation in math means that the equation is true. The number of atoms that are in the reactants must be the same as the number of atoms that are in the products.
Before we get into the meat of our Solving Equations unit we are going to quickly take a look at balancing equations. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products. A balanced chemical equation is characterized by each of the atoms of the reactants and the products being equal to each other.
The arrow indicates that the chemicals called. And there are two hydrogen atoms and one oxygen odd on the right side or the products out of this reaction. That both sides of the equation must be equal.
The atoms present in the reactants are also present in the products but are just rearranged. What does it mean for a chemical equation to be balanced. The number of different molecules has to be the same for both the reactants and products.
According to this law mass can neither be created nor be destroyed in a chemical reaction and obeying this law the total. We balance or solve equations but simplify or evaluate. Write a balanced molecular equation describing each of the following chemical reactions.
The law of conservation of matter except in nuclear reactions indicates that atoms can neither be created or destroyed. 123 If you add 1 toone side you get an imbalanced equation. A Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas b Gaseous butane CHe reacts with diatomic oxygen.
Theequals sign is a statement of fact such as. In above chemical equation number of hydrogen atoms on reactant side and product side is 2 whereas the number of chlorine atoms on both sides is also equal to 2. There are two arrows used.
Balancing the equation balancing the reaction conservation of charge and mass. Its important to remember that equations have an equal sign and expressions do not. It is important to make the equation balanced to make sure that the law of conservation of mass is not violated.
For example in this reaction Ive drawn here there are two hydrogen atoms into oxygen items on the reactions with the left side of this reaction. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. The number of atoms is the same for both the reactants and products.
The word equation is written correctly in the form of formula equation using symbols and molecular formula. As you begin solving equations in Algebra the very first thing that you will learn is that whatever you do you MUST keep your equation balanced. The chemical equation is said to be balanced when it contains equal number of atoms of different elements on the reactant side and the product side.
Chemical equations must be balanced to satisfy the law of conservation of matter that states that matter cannot be produced or destroyed in a closed system. What you do to one side of an equation you must do to the other in order for it to remain balanced. Think of every equation as a balanced scale.
What does it mean for a chemical equation to be balanced. For a chemical equation to be balanced the number of each type of the atom must be the same for both the reactants and the products. It means that the law of conservation of mass is obeyed.
The numbers placed in front of formulas to balance equations are called coefficients and they multiply all the atoms in a formula. The number of atoms of each element are counted on each side of the chemical equation. Think of an equation as a balance scale that must always be balanced.
The number of each type of atom is the same for both the reactants and products. The number of each type of atom is the same for both the reactants and products. A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side.
What does the arrow in a balanced chemical equation mean.

Sweetly Balanced Equations Equations Physical Science Lessons Middle School Chemistry

What Is A Balanced Equation In Chemistry Math Methods Chemical Equation Mental Math

Balance The Equation 1st Grade Math 1st Grade Math Worksheets First Grade Math

Balancing Equations Anchor Charts Used To Teach Students The Importance Of The Equal Sign And What It Means Gr Equations Teacher Activities Middle School Math

Balancing Chemical Equations Worksheet Chemistry Worksheets Chemical Equation Balancing Equations

Chemical Equations Balanced Or Not Equations Chemical Equation Chemical

How To Balance Chemical Equations 11 Steps With Pictures Chemical Equation Equations Chemistry Classroom

Balancing Chemical Equations Lesson Plan A Complete Science Lesson Using The 5e Method Of Instructi Chemical Equation Equations Balancing Equations Chemistry

Balanced Equations Anchor Chart Math Expressions First Grade Math Teaching First Grade

How To Balance Chemical Equations Chemical Equation Equations Chemistry Lessons

Balanced Chemical Equation For Cellular Respiration Meaning And Function Science Trends Cellular Respiration Chemical Equation Equation

Academicinspirational Phet Balancing Chemical Equations Worksheet Answers Phetbalancingact Chemistry Worksheets Chemical Equation Word Problem Worksheets

50 Balancing Equations Worksheet Answers Chemistry Chessmuseum Template Library Balancing Equations Chemical Equation Equations
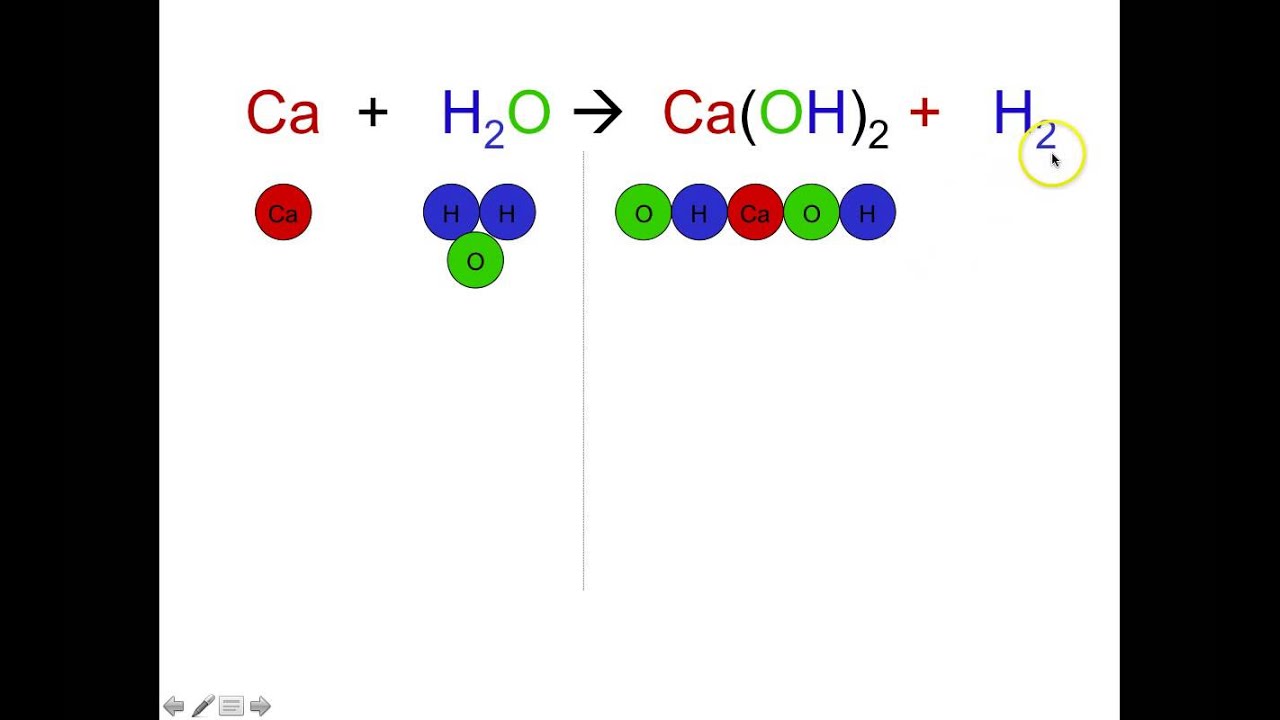
How To Balance Chemical Equations Simple Method For Beginners Chemical Equation Teaching Chemistry Chemistry Classroom

Balancing Chemical Equations Equations Quotes Chemical Equation Chemistry Worksheets

Balanced Equations Balance Scale Sheet Protector Dry Erase Practice Math Expressions Math Addition 1st Grade Math

Balance Chemical Equations Worksheet Balancing Equations Chemical Equation Balancing Equations Chemistry
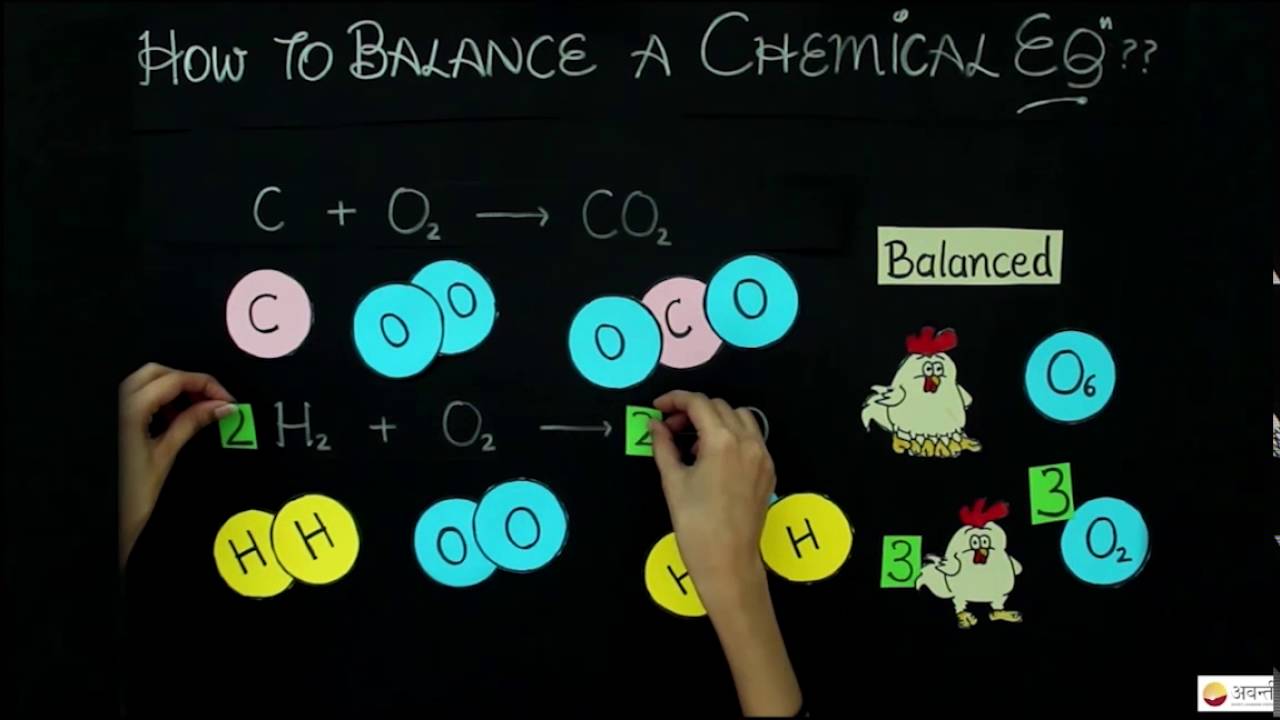
Balancing Simple Chemical Equations This Method Works Every Time Youtube Chemical Equation Chemistry Classroom Equations

How To Balance Chemical Equations 11 Steps With Pictures Chemical Equation Balancing Equations Balancing Equations Chemistry
Comments
Post a Comment